Double aromatic rings stabilize multications
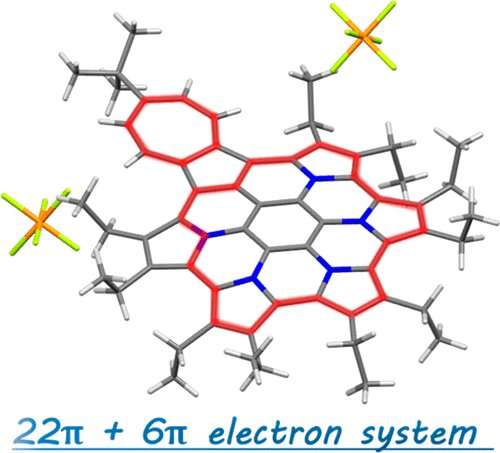
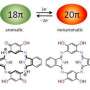
A redox active polycyclic aromatic hydrocarbon (PAHs) composed of azulene and pyrroles was developed by a research team at Ehime University. This nitrogen-embedded PAH contains two kinds of aromatic rings in the dicationic state which can stabilize cations by delocalization based on global aromaticity. The findings were published on March 5, 2019 in Organic Letters.
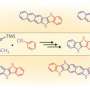
Redox active PAHs are especially important for applications such as organic electronics. Based on this background, the team reported pyrrole-fused azacoronene, i.e., hexapyrrolohexaazacoronene (HPHAC), where six pyrrole rings were fused to a coronene core. Due to the circularly connected pyrrole rings, oxidized species were reversibly obtained, exhibiting global aromaticity in the dicationic state by forming a 22π-electron conjugation.
In this study, pyrrole- and azulene-fused azacoronene was newly designed and synthesized. The electron-deficient seven-membered ring of azulene was expected to stabilize a cationic charge by forming an aromatic tropylium cation. In fact, crystal structure analysis, as well as DFT calculations, revealed both an aromatic 22π-electron conjugation and a tropylium cation (6π-electron conjugation). The control of charge resonance is an important design strategy in creating organic functional materials . The present contribution opens up the study of PAH chemistry.
More information: Yoshiki Sasaki et al. Synthesis and Redox Properties of Pyrrole- and Azulene-Fused Azacoronene, Organic Letters (2019). DOI: 10.1021/acs.orglett.9b00515
Journal information: Organic Letters
Provided by Ehime University